Advertisements
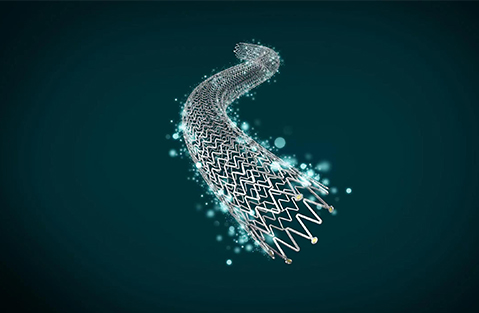

Peripheral Drug-eluting Stent System
| Price: | US$ 1300 |
|---|---|
| Minimum Order: | |
| Payment Terms: | 100% T/T in advance |
| Port of Export: |
Product Details
| Model No.: | Brand Name: |
|---|
| Certification: | |
|---|---|
| Specification: |
OVERVIEW
Peripheral Drug-eluting Stent System ZENFLEX¬ Pro Peripheral Drug-eluting Stent System, the 3rd peripheral drug-eluting stent in the world, is designed especially for complex lesions and restenotic symptomatic lesions. |
Packaging & Delivery
| Packaging: | |
|---|---|
| Delivery/Lead Time: | |
| Production Capacity: |
Product Description
OVERVIEW
Peripheral Drug-eluting Stent System
ZENFLEX¬ Pro Peripheral Drug-eluting Stent System, the 3rd peripheral drug-eluting stent in the world, is designed especially for complex lesions and restenotic symptomatic lesions.
PRODUCT FEATURES
Peripheral Drug-eluting Stent System
Based on ZENFLEX Peripheral stent platform
UNIQUE STENT DESIGN
Open helical structure
Segmented MicroMesh design
Offset Peak-Valley design
Results in superior flexibility and great radial force of the stent
Promises immediate wall apposition and precise stent placement
Polymer free Coating
The special ZENFLEX¬ Pro coating technology is a ultrasound spray crystalline paclitaxel.
Uses a special auxiliary gas to recrystallize the drug coating and obtain a stable uniform drug coating layer bonded on the stent surface.
Free from any polymer, adhesive, or excipient, while eliminating the potential risks that polymers may cause.
Provides the effective Paclitaxel tissue concentration within 90 days, and the stent converts into bare stent with complete drug delivery after 180 days
ORDERING INFORMATION
Usable Length Usable Length Stent Length Stent Diameter Sheath
(80cm) (125cm) (mm) (mm)
ZX05020080A ZX05020125A 20 5 6F
ZX05030080A ZX05030125A 30
ZX05040080A ZX05040125A 40
ZX05060080A ZX05060125A 60
ZX05080080A ZX05080125A 80
ZX05100080A ZX05100125A 100
ZX05120080A ZX05120125A 120
ZX05150080A ZX05150125A 150
ZX06020080A ZX06020125A 20 6 6F
ZX06030080A ZX06030125A 30
ZX06040080A ZX06040125A 40
ZX06060080A ZX06060125A 60
ZX06080080A ZX06080125A 80
ZX06100080A ZX06100125A 100
ZX06120080A ZX06120125A 120
ZX06150080A ZX06150125A 150
ZX07020080A ZX07020125A 20 7 6F
ZX07030080A ZX07030125A 30
ZX07040080A ZX07040125A 40
ZX07060080A ZX07060125A 60
ZX07080080A ZX07080125A 80
ZX07100080A ZX07100125A 100
ZX07120080A ZX07120125A 120
ZX07150080A ZX07150125A 150
We are one of leading neurovascular medical device companies in the peripheral and neurovascular interventional medical device market in China. As a fully integrated medical device company, zylox tonbridge medical technology has strong R&D and manufacturing capabilities, multiple specialized technology platforms, and proven commercialization capabilities. Led by our talented management team, we strive to provide best medical services and cutting-edge technical solutions to physicians and patients in China and around the world.

|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | ZYLOX-TONBRIDGE MEDICAL TECHNOLOGY CO., LTD. | ||
| City/State | Hangzhou, Zhejiang | Country: |
China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | 2012 |
| Member Since: | 2022 | Contact Person | Zyloxtb com |
SUPPLIER PROFILE
City/State/Country -
Hangzhou, Zhejiang
China 

Business Type -
Export - Manufacturer / Trading Company
Established -
2012
Member Since -
2022
Contact Person -
Zyloxtb com



