Advertisements


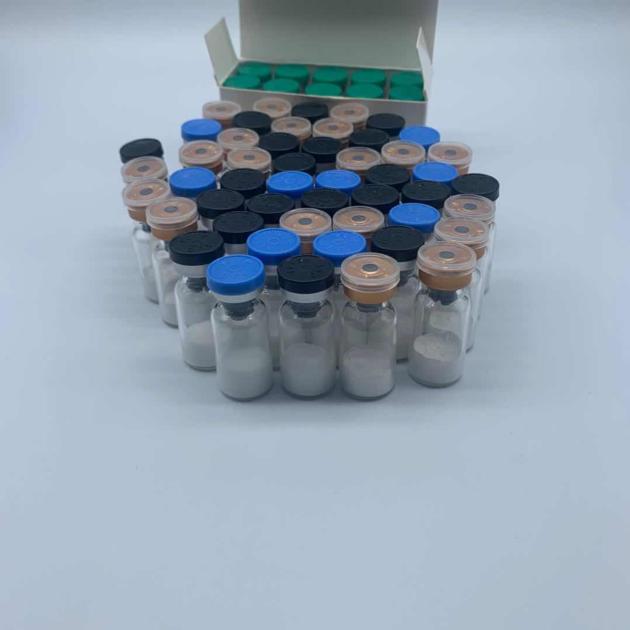
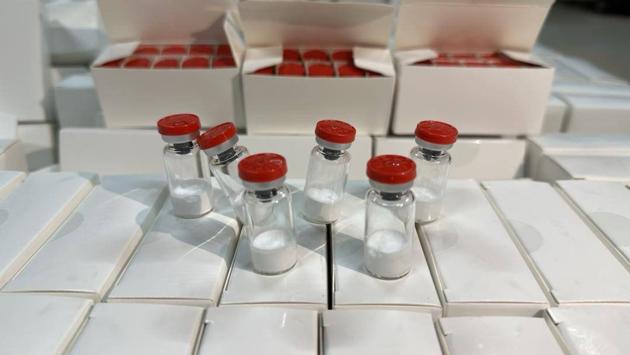
I.Weight loss peptide semaglutide 99% purity
| Price: | US$ 75 / Box |
|---|---|
| Minimum Order: | 1 box |
| Payment Terms: | bitcoin , westunion,moneygram,usdt |
| Port of Export: | Tianjing shanghai Guangzhou |
Product Details
| Model No.: | 910463-68-2 | Brand Name: | QL |
|---|
| Certification: | |
|---|---|
| Specification: |
2mg/vial*10vial/kit
5mg/vial*10vial/kit 10mg/vial*10vial/kit 1g |
Packaging & Delivery
| Packaging: | Select best ways according to quantity and safety degree,professional and experienced. |
|---|---|
| Delivery/Lead Time: | 10-15days |
| Production Capacity: | 1000 |
Product Description
Product Description
Item Specifications
semaglutide
Product Name
Appearance White freeze-dried powder
Purity 99%min
Boiling point 1265.3oC at 760 mmHg
Density 1.27 g/cm3
Refractive index 1.638
Single Impurity (HPLC) 1.0%max
Amino Acid Composition ¯10% of theoretical
Peptide Content (N%) ≥80.0%
Water Content(Karl Fischer) ≤6.0%
Acetate Content (HPIC) ≤12.0%
MS(ESI) Consistent
Mass Balance 95.0~105.0%
Semaglutide is currently approved as a type 2 diabetes (T2D) treatment. In combination with exercise and diet, it is proven to lower blood sugar levels and improve glycemic control.
Semaglutide is a derivative of the naturally occurring GLP-1, a peptide known to lower blood sugar levels and enhance insulin secretion. Research shows that Semaglutide may also improve heart, liver, and lung function while helping to slow or prevent the effects of Alzheimer’s disease. Semaglutide has been shown to significantly decrease appetite by delaying gastric emptying and reducing intestinal motility. Glucagon-Like Peptide-1 (GLP-1) Analog Shown to Stimulate Insulin and Suppress Glucagon Secretion in a Glucose-Dependent Manner.
Semaglutide subcutaneous injection (Ozempic) and oral tablets (Rybelsus) are used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM). Semaglutide oral tablets demonstrated CV safety by meeting the primary endpoint of non-inferiority for the composite MACE endpoint; the proportion of patients who experienced at least one MACE was 3.8% with semaglutide oral tablets and 4.8% with placebo.1 However, semaglutide oral tablets are not approved for the reduction of CV events




|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | Hebei zhuojiang Import and Export Technology Co., LTD | ||
| City/State | ShijiaZhuang, Hebei | Country: |
China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | 1600 |
| Member Since: | 2023 | Contact Person | Miya h |
SUPPLIER PROFILE
City/State/Country -
ShijiaZhuang, Hebei
China 

Business Type -
Export - Manufacturer / Trading Company
Established -
1600
Member Since -
2023
Contact Person -
Miya h



