Advertisements


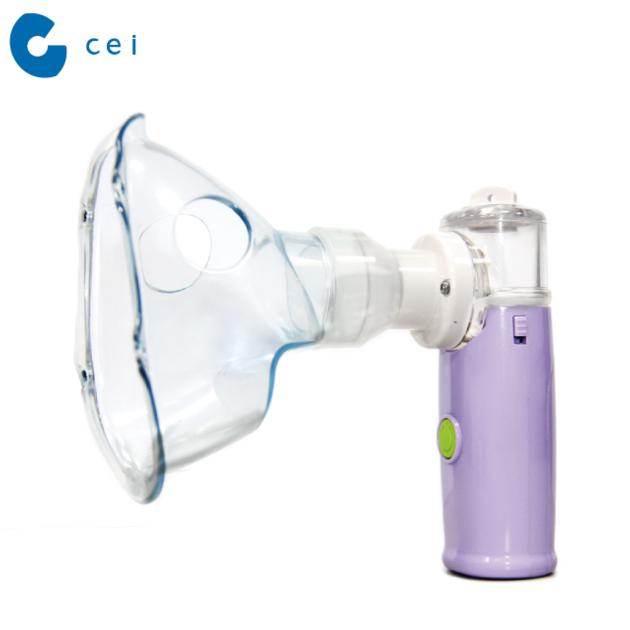
2018 FDA CE ISO Approved Ultrasonic Portable Nebulizer with mask Nebulizer
| Price: | US$ 22 |
|---|---|
| Minimum Order: | 100 |
| Payment Terms: | L/C,T/T,Western Union, MoneyGram, Paypal |
| Port of Export: | Taipei |
Product Details
| Model No.: | CE-333 | Brand Name: | CEI |
|---|
| Certification: | |
|---|---|
| Specification: |
SPECIFICATION
Method of Operation Ultrasonic (Atomize by Micromesh) Size Approx. 26 mm ( L ) x 35 mm ( W ) x 98 mm ( H ) Weight Approx. 46g (Excluding battery) Power 3V DC (Two “AAA”1.5V alkaline batteries) Power consumption Approx. 0.6W Vibrating Frequency Approx. 117kHz Spray rate > 0.25mL/min Particle Size MMAD 3 - 5 μm capacity Approx. 8 mL Maximum Approx. 1 mL Minimum battery life 4 days if used daily for 15 minutes. Epected Service Life Nebulizer main unit: 1 Year Medication cup: 3 Months Operating Conditions 10 ℃ ~ 40℃(50 ℉ ~ 104 ℉), 30 ~ 85% R.H. Storage / Transportation Conditions -20 ℃ ~ 70℃(-4 ℉ ~ 158 ℉), <= 85% R.H. Accessory main unit (Include battery cover) x1 medication cup x1 mask (L) x 1 mask (S) x 1 10mL (Medicine cup) x 1 ‘AAA’ alkaline 1.5V Battery x 2 zippered pouch x 1 Instruction Manual x 1 |
Packaging & Delivery
| Packaging: | Standard Packaging/ Customized |
|---|---|
| Delivery/Lead Time: | |
| Production Capacity: | 10000 per month |
Product Description
INTRODUCTION
An aerosol respiratory therapy of asthma nebulizer atomizes liquid (normal saline), and sprays it into the user’s airway in order to keep the respiratory tract unobstructed, moisten the respiratory tract, and dilute sputum. A major task of nebulizer or inhaler would be its efficiency to deliver the medication to the lower respiratory. Furthermore, the efficiency is tightly correlate to the particle size. CE-333 Ultrasonic Nebulizer is featured in its extremely fine particle size, less than 4 microliter.
APPLICATION
For adult and pediatric patients suffering from Asthma or Chronic Obstructive Pulmonary Disease (COPD) such as; emphysema and chronic bronchitis, or other respiratory, pulmonary or brochial diseases that are characterized by obstruction to air flow, simply used in throat moisturizing or delivering the medication to the expected positition. *NOTE* This is a single patient device. Do not allow multiple patients to use the same nebulizer.
FEATURE
Extremely Quiet
Low Power Consumption
Ergonomic handle
One button operation
4 microliter particle size
Mesh-Ultrasonic Technology
Colorful Appearance
Micro-pump technology
Low medicine residue
Allow operation in all directions, up side down or slant for around 10 seconds.
Completely silent in operation
Low power consumption
Automatic turn off when medicine is depletion
10 minutes to atomize 7ml medicine (high speed rate)
Electronic aerosol generator
MMAD particle size: Less than 4 microliter
Capacity of medicine cup: 10ml





|
SUPPLIER PROFILE
|
|||
|---|---|---|---|
| Company: | C.E.I. Technology Inc. | ||
| City/State | Taoyuan County, | Country: |
Taiwan - Province of China 
|
| Business Type: | Export - Manufacturer / Trading Company | Established: | NA |
| Member Since: | 2014 | Contact Person | Lewis Li |
SUPPLIER PROFILE
City/State/Country -
Taoyuan County,
Taiwan - Province of China 

Business Type -
Export - Manufacturer / Trading Company
Established -
NA
Member Since -
2014
Contact Person -
Lewis Li



